What is the Difference Between Heat and Thermal Energy?
The difference between heat and thermal energy is in their kinematics; where heat entails energy flow from one body or system to another, while thermal energy causes molecules or components of a body or system to vibrate or rotate about a fixed point.
This article shows that the difference between heat and thermal energy is a function of multiple factors, and discusses some of these alongside other related inquiries.
How is Thermal Energy Different from Heat?
Thermal energy is different from heat in terms of fundamental concept, level of operation (molecular, systemic), effect on systems, and thermodynamic implications.
Although thermal energy and heat may seem similar, they have distinct differences. Understanding these differences is crucial in comprehending the nature of heat and thermal energy.
*Firstly, thermal energy refers to the internal energy of a system due to the random motion and vibration of its molecules or particles. It is a measure of the total kinetic and potential energy of the particles within a substance. On the other hand, heat is the transfer of thermal energy from one body or system to another. Heat is the energy in transit, moving from a region of higher temperature to a region of lower temperature.
*Secondly, the level of operation distinguishes thermal energy from heat. Thermal energy operates at the molecular level, involving the motion and interaction of individual particles within a substance. It is associated with the microscopic behavior of the system. In contrast, heat operates at a systemic level, involving the transfer of energy between different bodies or systems. It focuses on the macroscopic behavior and energy flow between systems.
*Furthermore, the effect on systems sets thermal energy apart from heat. Thermal energy causes the molecules or components of a body or system to vibrate or rotate about a fixed point. It contributes to the internal energy and temperature of the system. Heat, on the other hand, leads to a change in temperature or phase of a substance when it is transferred from one system to another. It affects the overall energy balance and equilibrium of the systems involved.
*Lastly, the thermodynamic implications of thermal energy and heat differ. Thermal energy is a form of internal energy that can be converted into other forms, such as mechanical work or electrical energy. It is a fundamental concept in thermodynamics, playing a crucial role in understanding energy transformations. Heat, on the other hand, is a mode of energy transfer that follows the laws of thermodynamics, specifically the principles of conservation of energy and the second law of thermodynamics.
Is Heat the Same as Thermal Energy?
While both describe thermodynamic processes, heat is not the same as thermal energy, but is rather a measure of the flow of thermal energy from one body or system to another. Heat can be thought of as the transfer of thermal energy, rather than the energy itself.
*Heat travels through vibrations caused by thermal energy. When there is a temperature difference between two bodies or systems, thermal energy flows from the body or system with higher temperature to the one with lower temperature. This transfer of thermal energy is what we refer to as heat. It is the movement of energy from one place to another, driven by the temperature difference.
*Heat is a form of energy in transit. It is the energy that is being transferred from one object to another due to a temperature difference. Thermal energy, on the other hand, is the total energy of the particles within a substance. It includes both the kinetic energy of the particles due to their motion and the potential energy due to their position or arrangement. Thermal energy is the internal energy of a system, while heat is the energy being transferred between systems.
*Another way to understand the difference is to consider the level of operation. Thermal energy operates at the molecular level, involving the motion and interaction of individual particles within a substance. It is associated with the microscopic behavior of the system. Heat, on the other hand, operates at a systemic level, involving the transfer of energy between different bodies or systems. It focuses on the macroscopic behavior and energy flow between systems.
*Generally, heat and thermal energy are related but distinct concepts. Thermal energy refers to the internal energy of a system, while heat is the transfer of thermal energy from one body or system to another. Heat is the flow of thermal energy, driven by a temperature difference. Understanding this distinction is crucial in comprehending the nature of heat and thermal energy and their roles in thermodynamic processes.
Heat Vs Thermal Energy
Heat vs thermal energy can be compared and analyzed using factors like energy transfer, source(s) of energy, nature of energy dynamics, implications for thermodynamic systems, and interdependence; for each concept (heat and thermal energy) respectively.
*Energy Transfer
Heat is the transfer of thermal energy from one body or system to another. It is the movement of energy due to a temperature difference. Thermal energy, on the other hand, refers to the total energy of the particles within a substance. It includes both the kinetic and potential energy of the particles. While heat involves the transfer of energy, thermal energy is the energy contained within a system.
*Source(s) of Energy
Heat is produced from thermal energy. When there is a temperature difference between two bodies or systems, thermal energy flows from the body or system with higher temperature to the one with lower temperature. This transfer of thermal energy is what we refer to as heat. Thermal energy, on the other hand, is the internal energy of a system and can be obtained from various sources such as chemical reactions, nuclear reactions, or electrical energy.
*Nature of Energy Dynamics
Heat is transitory in nature. It is the energy that is being transferred from one object to another. Thermal energy, on the other hand, is internal or contained within a system. It represents the total energy of the particles and is not dependent on the transfer of energy.
*Implications for Thermodynamic Systems
Heat implies a change in inertia. When heat is transferred to a system, it can cause a change in the system’s temperature, phase, or state. Thermal energy, on the other hand, does not imply a change in inertia. It represents the internal energy of the system and is responsible for the system’s overall behavior.
*Interdependence
Heat and thermal energy are interdependent concepts. Heat is the transfer of thermal energy, and thermal energy is the source of heat. Without thermal energy, there would be no heat transfer. Similarly, without heat transfer, thermal energy would remain contained within a system.
1. Heat Occurs Due to Difference in Temperature
Heat and thermal energy are closely related concepts, but they differ in their dependence on temperature differences. Heat is a form of energy transfer that occurs when there is a temperature difference between two bodies or systems. It is the movement of thermal energy from a region of higher temperature to a region of lower temperature.
*Heat is the transfer of thermal energy from one body to another. It occurs when there is a temperature gradient, meaning there is a difference in temperature between the two bodies. This temperature difference drives the flow of thermal energy, resulting in the transfer of heat.
*On the other hand, thermal energy does not depend on temperature differences. It refers to the total energy of the particles within a substance, including both their kinetic and potential energy. Thermal energy is an intrinsic property of a system and is not influenced by the presence or absence of temperature differences.
Understanding the distinction between heat and thermal energy is crucial in the evaluation of energy dynamics and thermodynamic systems. Heat transfer relies on temperature differences to occur, while thermal energy remains constant within a system regardless of temperature variations.
2. Heat is Produced from Thermal Energy
Heat is the result of the flow of thermal energy. When there is a temperature difference between two bodies or systems, thermal energy moves from the region of higher temperature to the region of lower temperature, resulting in the transfer of heat. This transfer occurs through various mechanisms such as conduction, convection, and radiation.
*Conduction is the transfer of heat through direct contact between particles. When particles with higher thermal energy collide with particles with lower thermal energy, the energy is transferred, causing an increase in temperature in the colder region.
*Convection involves the transfer of heat through the movement of fluids or gases. As particles gain thermal energy, they become less dense and rise, while cooler particles sink. This creates a continuous circulation of thermal energy, leading to the transfer of heat.
*Radiation is the transfer of heat through electromagnetic waves. Unlike conduction and convection, radiation does not require a medium to propagate. Objects with higher thermal energy emit electromagnetic waves, which can be absorbed by objects with lower thermal energy, resulting in the transfer of heat.
The production of heat from thermal energy is driven by the vibrations of particles within a substance. As particles vibrate, they generate kinetic energy, which is a form of thermal energy. These vibrations can be caused by various factors such as the absorption of energy from an external source, chemical reactions, or changes in phase.
Understanding the relationship between heat and thermal energy is essential in various fields, including engineering, physics, and thermodynamics. By comprehending how thermal energy is converted into heat and transferred between systems, scientists and engineers can design efficient heat transfer systems, optimize energy usage, and develop innovative technologies.
The next section explores another key difference between heat and thermal energy: the transitory nature of heat and the internal or contained nature of thermal energy.
3. Heat is Transitory, Thermal energy is Internal or Contained
Heat and thermal energy differ in terms of their transitory nature and internal containment. While heat is a transient form of energy that flows from a region of higher temperature to a region of lower temperature, thermal energy is internal to a system and remains relatively stable.
* Heat, as mentioned earlier, is the result of the flow of thermal energy. It is a transitory form of energy that moves from one place to another due to a temperature difference. When there is a higher temperature in one region and a lower temperature in another, heat transfers from the hotter region to the colder region until thermal equilibrium is reached.
* On the other hand, thermal energy is contained at the molecular level within a substance or system. It represents the total kinetic and potential energy of the particles that make up the system. This energy is internal to the system and does not necessarily involve the transfer of heat. Thermal energy remains relatively stable unless there is an external influence or change in the system.
Understanding this distinction is crucial in various applications. For example, in thermodynamics, engineers design systems to efficiently transfer heat from one place to another. They focus on optimizing the flow of heat while minimizing energy losses. In contrast, understanding the internal thermal energy of a system helps scientists analyze its stability, behavior, and potential for work.
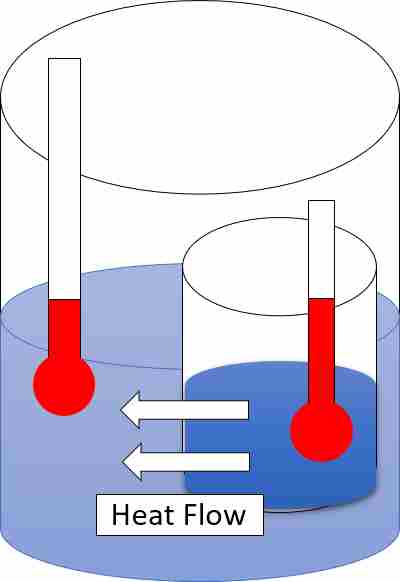
4. Heat Implies Change in Inertia, Thermal Energy Does Not
This differentiation between heat and thermal energy lies in their effects on inertia. While heat implies a change in inertia, thermal energy does not directly affect inertia.
* Heat, as a form of energy transfer, involves the movement of particles and the transfer of kinetic energy. When heat is added to a system, the particles gain energy and their motion increases. This increase in motion affects the inertia of the system, as the particles now have more energy to overcome resistance and change their state of motion. For example, when heat is applied to a solid, the increased kinetic energy of the particles allows them to vibrate more vigorously, leading to a change in the solid’s shape or state.
* On the other hand, thermal energy represents the total energy of the particles within a system, including both kinetic and potential energy. It does not directly cause a change in inertia because it is already internal to the system. Thermal energy is the sum of the energies of the individual particles, and it remains relatively constant unless there is an external influence or change in the system.
The understanding of this distinction is important in various fields, such as physics and engineering. For instance, in thermodynamics, the study of heat and energy transfer, engineers consider the effects of heat on the inertia of systems. They analyze how heat can cause changes in the motion and behavior of objects, leading to thermal expansion, phase changes, or other thermodynamic phenomena.
5. Heat is Systemic, Thermal Energy is Molecular
This differentiation between heat and thermal energy lies in their systemic and molecular nature. Understanding this distinction is crucial in comprehending the behavior and characteristics of these two forms of energy.
* Heat, as a systemic form of energy, refers to the transfer of energy between objects or systems due to a difference in temperature. It involves the movement of energy from a region of higher temperature to a region of lower temperature. Heat can be transferred through conduction, convection, or radiation. For example, when you touch a hot object, heat is transferred from the object to your hand, resulting in a sensation of warmth.
* On the other hand, thermal energy is molecular in nature. It represents the total energy of the individual particles within a system, including both kinetic and potential energy. Thermal energy is associated with the random motion and vibrations of molecules and atoms. It is a measure of the internal energy of a substance and is directly related to its temperature. For instance, when you heat a substance, the thermal energy of its molecules increases, causing the substance to expand or change state.
The systemic nature of heat means that it involves the transfer of energy between objects or systems, while thermal energy is confined to the molecular level within a system. Heat can be thought of as the energy in transit, whereas thermal energy is the energy contained within the system itself.
6. Presence of Thermal Energy is a Prerequisite for Heat Flow
The presence of thermal energy is essential for the flow of heat, but the reverse is not true. Thermal energy is not dependent on heat but can be derived from kinetic energy.
* Thermal energy, as mentioned earlier, is the total energy of the individual particles within a system. It encompasses both kinetic energy, which is the energy of motion, and potential energy, which is the energy stored in the position or configuration of the particles. This means that even in the absence of heat transfer, the particles in a system still possess thermal energy due to their inherent motion and vibrations.
* Heat flow, on the other hand, occurs when there is a difference in temperature between two objects or systems. It is the transfer of thermal energy from a region of higher temperature to a region of lower temperature. Heat flow is driven by the presence of thermal energy, as it is the molecular motion and vibrations that allow for the transfer of energy.
Therefore, the presence of thermal energy is a prerequisite for heat flow to occur. Without thermal energy, there would be no energy available for transfer, and thus no heat flow. However, it is important to note that thermal energy can exist even in the absence of heat transfer, as it is derived from the kinetic energy of the particles within a system.
7. Heat is Transferred by Conduction/Convection/Radiation; Thermal Energy by Molecular Vibration
This section explores how heat and thermal energy are transferred and the mechanisms involved. Heat is transferred through conduction, convection, and radiation, while thermal energy is transferred through molecular vibration.
* Conduction is the transfer of heat through direct contact between two objects or substances. When objects are in contact, the particles with higher kinetic energy transfer their energy to particles with lower kinetic energy, resulting in the transfer of heat. For example, when you touch a hot stove, heat is transferred from the stove to your hand through conduction.
* Convection is the transfer of heat through the movement of fluids or gases. In convection, the heated particles become less dense and rise, while the cooler particles sink. This creates a continuous circulation of fluid or gas, transferring heat in the process. An example of convection is the heating of a room through a radiator, where the hot air rises and circulates, warming the entire space.
* Radiation is the transfer of heat through electromagnetic waves. Unlike conduction and convection, radiation does not require a medium to transfer heat. Heat is emitted in the form of electromagnetic waves, such as infrared radiation, and can travel through empty space. This is how the Sun’s heat reaches the Earth, as it travels through the vacuum of space.
On the other hand, thermal energy is transferred through molecular vibration. The particles within a substance vibrate and move, possessing kinetic energy.
This molecular motion is responsible for the transfer of thermal energy. When two objects or substances come into contact, the particles with higher kinetic energy transfer some of their energy to particles with lower kinetic energy, resulting in the transfer of thermal energy.

|
Heat |
Thermal Energy |
|
Heat is a product of thermal energy-induced vibrations |
Thermal energy is not always a product of heat |
|
Occurs due to differences in temperature |
Is not dependent on temperature differences |
|
Is a transitory quantity |
Is contained in/by a body/system |
|
Always implies a change in inertia |
Does not imply a change in inertia |
|
Is a systemic quantity (that is, operates across systems) |
Is a molecular quantity |
|
Thermal energy is a prerequisite for heat flow |
Raw heat is not necessarily required for thermal energy. Kinetic energy can serve as a parent energy form. |
|
Is transferred by conduction, convection, radiation |
Is transferred by molecular vibration |
Similarities and Relationship Between Heat and Thermal Energy
Similarities and relationship between heat and thermal energy include their thermodynamic contexts, unit(s) of measurement, transformative impact on molecules and bodies.
* Both heat and thermal energy are concepts within the field of thermodynamics. They are related to the study of energy and its transformation in various systems.
* Heat and thermal energy are measured using the same unit of measurement, which is the joule (J). This unit quantifies the amount of energy transferred or possessed by a system.
* Both heat and thermal energy have the ability to transform the molecules and bodies they interact with. When heat is added to a substance, the kinetic energy of its particles increases, causing them to move faster and vibrate more vigorously. Similarly, thermal energy is responsible for the molecular motion within a substance, which affects its temperature and physical properties.
* Heat and thermal energy can be transferred from one object to another. This transfer can occur through conduction, convection, and radiation, as discussed in the preceding section. In all cases, the transfer of heat or thermal energy involves the movement of energy from a region of higher temperature to a region of lower temperature.
* Both heat and thermal energy play a crucial role in various natural and industrial processes. They are involved in phenomena such as phase changes (e.g., melting, boiling, condensation), chemical reactions, and the operation of heat engines.
* Heat and thermal energy are interconnected and can be converted into other forms of energy. For example, heat can be converted into mechanical work in a heat engine, such as a car engine or a power plant turbine. Similarly, thermal energy can be converted into electrical energy through the use of thermoelectric devices.
* The relationship between heat and thermal energy is such that heat is a form of energy transfer, while thermal energy refers to the internal energy possessed by a substance. Heat can be thought of as the flow of thermal energy from one object to another.
FAQs
1. What is the Relationship Between Heat and Thermal Energy?
The relationship between heat and thermal energy is that heat is a form of energy transfer, while thermal energy refers to the internal energy possessed by a substance. Heat can be thought of as the flow of thermal energy from one object to another. When heat is added to a substance, the kinetic energy of its particles increases, causing them to move faster and vibrate more vigorously. This increase in kinetic energy leads to an increase in the substance’s temperature and its thermal energy.
2. When the Temperature of an Object Decreases, What Has Happened?
When the temperature of an object decreases, it means that the average kinetic energy of its particles has decreased. In other words, the particles are moving slower and vibrating less vigorously. This decrease in kinetic energy results in a decrease in the object’s thermal energy. It is important to note that the decrease in temperature does not imply a loss of thermal energy, but rather a decrease in the amount of thermal energy present in the object.
3. What Happens to the Kinetic Energy of Particles When Heat is Added?
When heat is added to a substance, the kinetic energy of its particles increases. This increase in kinetic energy is a result of the transfer of energy from a region of higher temperature to a region of lower temperature. As the particles absorb the heat energy, they gain more energy and begin to move faster and vibrate more vigorously. This increase in kinetic energy leads to an increase in the substance’s temperature and its thermal energy.
4. What is the Difference Between Enthalpy and Thermal Energy?
Enthalpy and thermal energy are related concepts, but they have different meanings. Enthalpy is a thermodynamic property that represents the total heat content of a system. It includes both the internal energy of the system and the work done by or on the system. On the other hand, thermal energy refers specifically to the internal energy possessed by a substance due to the motion and vibration of its particles. While both enthalpy and thermal energy are measures of energy, enthalpy takes into account the work done by or on the system, while thermal energy focuses solely on the internal energy of the substance.
5. What is the Difference Between Heat and Thermodynamics?
Heat and thermodynamics are related concepts within the field of thermodynamics, but they have different meanings. Heat refers to the transfer of energy from a region of higher temperature to a region of lower temperature. It is a form of energy transfer.
On the other hand, thermodynamics is the branch of physics that deals with the study of energy and its transformations in various systems. It encompasses the study of heat, work, and the relationships between different forms of energy. While heat is a specific type of energy transfer, thermodynamics is a broader field that encompasses the study of energy and its transformations in general.
6. In Which Direction Does Heat Always Flow?
Heat always flows from a region of higher temperature to a region of lower temperature. This is known as the second law of thermodynamics. The flow of heat occurs until thermal equilibrium is reached, which is when the temperatures of the two regions are equal. In other words, heat flows in the direction that tends to equalize the temperatures of the objects or systems involved.
7. What Factors Determine the Thermal Properties of a Material?
The thermal properties of a material are determined by several factors. These include the specific heat capacity, thermal conductivity, and thermal expansion coefficient of the material.
– Specific heat capacity is a measure of the amount of heat energy required to raise the temperature of a given amount of the material by a certain amount. It is a property that is unique to each material and is dependent on its molecular structure.
– Thermal conductivity is a measure of how well a material conducts heat. It determines how quickly heat can be transferred through the material. Materials with high thermal conductivity, such as metals, are good conductors of heat, while materials with low thermal conductivity, such as insulators, are poor conductors of heat.
– Thermal expansion coefficient is a measure of how much a material expands or contracts when its temperature changes. It determines how much the dimensions of the material change with temperature. Materials with high thermal expansion coefficients expand more with temperature changes, while materials with low thermal expansion coefficients expand less.
These factors, along with other material-specific properties, determine how a material responds to changes in temperature and its ability to conduct or resist the flow of heat.
8. How Can You Calculate Changes in Thermal Energy?
Changes in thermal energy can be calculated using the equation:
ΔQ = m * c * ΔT
Where:
– ΔQ is the change in thermal energy
– m is the mass of the substance
– c is the specific heat capacity of the substance
– ΔT is the change in temperature
This equation allows you to calculate the amount of heat energy gained or lost by a substance when its temperature changes. By knowing the mass of the substance, its specific heat capacity, and the change in temperature, you can determine the change in thermal energy.
9. Why is Heat Measured in Joules?
Heat is measured in joules because it is a form of energy, and the joule is the standard unit of measurement for energy. The joule is a derived unit in the International System of Units (SI) and is defined as the amount of energy transferred or work done when a force of one newton acts over a distance of one meter. Since heat is a form of energy transfer, it is measured in joules to quantify the amount of energy transferred during a heat transfer process.
10. How Does Thermal Energy Relate to Temperature During Condensation?
During condensation, thermal energy is released from a substance as it changes from a gas to a liquid state. The release of thermal energy causes the substance to cool down and its temperature to decrease.
This is because the energy that was previously in the form of kinetic energy of the gas molecules is now being released as heat energy to the surroundings. As a result, the thermal energy of the substance decreases, leading to a decrease in temperature. The condensation process is an example of how thermal energy and temperature are interconnected and how changes in thermal energy can affect the temperature of a substance.