Closed System Definition, Theory and Examples Explained
Closed system is any system which exchanges its energy resources with the external environment, but does not allow any exchange of matter.
This article discusses closed system definition, theory and examples, as outlined below;
-Closed System Definition: 4 Ways to Define a Closed System
Closed System Definition: 4 Ways to Define a Closed System
A closed system is any system that exchanges only energy with its surroundings, while retaining its original mass.
The above is a most basic closed system definition. Below is a modified version of it, which specifically describes a closed system in thermodynamics;
A closed system is any entity or body having definite boundaries, across which the transfer of matter is not possible, so that only energy of various forms can be exchanged with the external environment [1].
Another term which a closed system is called is limited system. However, this term is more usable within context involving IoT technology, and economics. Closed system is acceptable in chemical and physical contexts involving mechanics and thermodynamics.
The two main components found in a closed system are matter and energy.
The next closed system definition, given below, outlines the meaning of a closed system with examples;
A closed system is best described as a partially-isolated physical system that can exchange only energy with its surroundings, but does not allow transfer of matter. Some examples of closed systems include; sealed heater, composter, nuclear reactor, water bottle, and the Earth.
It must be noted that the above examples represent closed systems within the context of thermodynamics, which is the most common context in which a closed system is evaluated.
For other contexts like computer technology, economics and society; examples of closed system can include user-restricted data management systems, private organizations, and independent policies.
In chemistry, a closed system can be exemplified by a system in which chemical reactions release only thermal energy to the environment, while total mass of the reactants is conserved in the products.
The final closed system definition which is given below, describes closed system according to chemical principles;
A closed system is a system that prevents the escape of reactants and products, but allows energy to be exchanges between its interior, and the external environment [2].
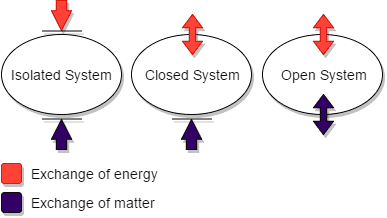
Closed system is called partially-isolated system, when described with respect to other types of thermodynamic systems; which are open and isolated systems.
An open system is one which exchanges both matter and energy with its surroundings, while an isolated system is one which exchanges neither matter nor energy with its surroundings.
Closed systems fall between the other two types in terms of basic characteristics, and exhibit the tendency to prevent interaction with the external environment, like isolated systems, while allowing some of its components (namely; energy), to be transferred.

Closed System Theory
Closed system theory is used to describe socioeconomic concepts like organizational behavior and social structural management.
The closed system theory is of the opinion that self-sustainability at the family, organizational or individual level, depends on the ability to resist the impact of external factors and changes by establishing an impervious social barrier [3].
In organizations, the closed system theory describes a state whereby an organization is able to exist with minimal influence from other entities within its socioeconomic ecosystem; including industry stakeholders, policies, and competitors.
This contrasts with the theory of open systems, which describes the dynamic of input an output between an entity and its surroundings, where the exchange may include intellectual, economic or thermodynamic resources.
In mechanics, closed system theory is used to describe bodies or systems that exchange various forms of energy with other bodies or systems, but do not lose their internal mass in the process.
Examples of Closed Systems
Examples of closed systems in thermodynamics, chemistry, and socioeconomics are;
1). Subsurface hydrothermal reservoir
2). Nuclear reactor
3). Test tube containing exothermic reactants
4). Mobile solid body
5). Independent organization
6). Self-contained family
7). Sealed compost vessel
Conclusion
A closed system is any body, entity or unit that partially restricts social, economic or thermodynamic interaction with its surroundings.
Examples of closed systems are; sealed hydrothermal reservoir, pyrolytic reactor, test tube with exothermic reactants, mobile solid body, independent organization, self-contained body, and sealed compost vessel.
References
1). Dincer, I.; Cengel, Y. A. (2001). "Energy, Entropy and Exergy Concepts and Their Roles in Thermal Engineering." Entropy 3(3). Available at: https://doi.org/10.3390/e3030116. (Accessed 6 March 2023).
2). Helmenstine, A. M. (2019). "Scientific Definition of a Closed System in Thermodynamics." Available at: https://www.thoughtco.com/definition-of-closed-system-604929. (Accessed 6 March 2023).
3). Wirick, D. M.; Teufel-Prida, L. A. (2019). "Closed Systems in Family Systems Theory." In: Lebow, J., Chambers, A., Breunlin, D. (eds) Encyclopedia of Couple and Family Therapy. Springer, Cham. Available at: https://doi.org/10.1007/978-3-319-15877-8_249-1. (Accessed 6 March 2023).