5 Examples of Closed Systems Explained
Examples of closed systems are; sealed heater, water bottle, subsurface hydrothermal reservoir, compost vessel, and the Earth.
This article discusses the examples of closed systems, as follows;
1). Sealed Heater (as one of the Examples of Closed Systems)
Sealed heater, also called sealed heating system, sealed central heating system or sealed boiler, is a classic example of a thermodynamic closed system.
A sealed heating system is a system for generating and emitting thermal energy, whose physical boundaries seclude its fluid content from the environment or atmosphere.
From the above it can be deduced that a heating system is a closed system, when its internal mass remains constant at all times.
The difference between sealed and unsealed heating systems (also called open-vented systems) is that the former is equipped to regulate its fluid pressure internally, while the latter is de-pressurized by emitting its fluid content into an external system.
This external body in open-vented heating systems is the feed and expansion tank, which exchanges working fluid with the main heater to ensure that pressure is regulated for optimal energy efficiency and performance [2].
In the sealed boiler/heating system, an expansion vessel is also present, however it is installed so that it also stays within the confines of the main heater. When the working fluid in the boiler is heated and pressure increases, this pressure (along with temperature) is regulated by the migration (not release) of the vaporized working fluid into the expansion chamber.
The setup is very useful where water conservation (or conservation of any other working fluid) is necessary, as it ensures that fluid is not lost by the heating system at any time. It is also useful where simplicity and minimal maintenance are core objectives.
However, because of its restricted working conditions, the sealed heating system is not as efficient as open-vented system for large-scale heating purposes.
Sealed heating system clearly illustrates the basic attributes of a closed system, in terms of matter/mass conservation in combination with effective energy transfer.
2). Water Bottle
A water bottle is an example of a closed system, when it is sealed and composed of conductive material.
The most classic form of this is a sealed metallic bottle containing fluid.
An ideal thermos bottle is not a closed system, but rather is an isolated system, because it neither exchanges thermal energy nor fluid-mass with its surroundings.
However, in real-life scenarios, a thermos bottle is a closed system, because over an extended time period, it eventually releases its thermal energy to the surroundings, so that its fluid content cools down.
A hot water bottle is a closed system, while a hot water bottle that leaks is an open system, because it releases both heat and matter (fluid) to the external environment.

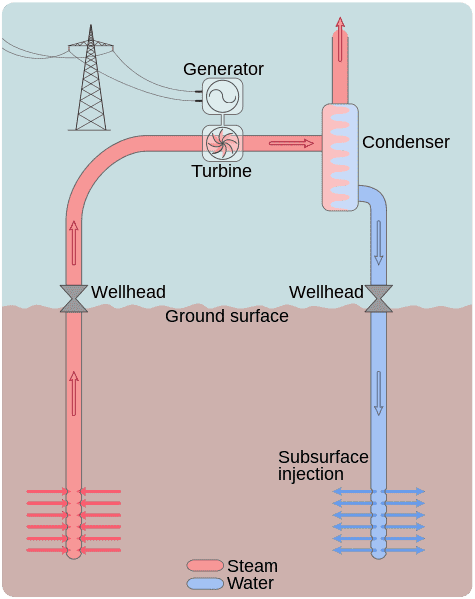
3). Subsurface Hydrothermal Reservoir (as one of the Examples of Closed Systems)
A hydrothermal system is one which has both hydro energy and thermal energy.
Hydrothermal systems work using the relationship between physical factors like pressure and temperature, to control the dynamics of fluids.
Characteristics of hydrothermal systems include significant fluid content, thermal energy, and definite physical boundaries.
The different types of hydrothermal systems are; subsurface, surface, open, closed, high and low-temperature hydrothermal systems.
The difference between hydrothermal and geothermal systems is that the primary components of hydrothermal systems are water and heat; while geothermal systems include water, heat, and earth materials like rocks, as primary components.
A hydrothermal system or reservoir can be described as a closed system, when it prevents the emission or introduction of water in any of its phases (liquid, gas, solid), across the physical boundaries of the reservoir.
The above describes a subsurface hydrothermal reservoir. Being underground, the physical boundaries of such a geologic system comprises of rocks and fluids, which allow heat to be transferred out of the reservoir, even to the surface.
Energy transfer for the subsurface hydrothermal reservoir occurs by conduction, radiation and convection. On the other hand, matter is not transferred, for as long as the reservoir is overlain and underlain by impervious rock materials.
The thermal energy in subsurface hydrothermal reservoirs is gotten primarily from magma [4], which in turn gains energy from nuclear radioisotopes in the Earth's core.
A subsurface hydrothermal reservoir becomes an open system when its physical boundaries are breached. This can occur due to human activities like the drilling of geothermal wells for power plants.
After drilling a well into the natural hydrothermal reservoir, a manmade closed system can still be created using fluid-pumping technology.
This is alternatively called a closed-loop geothermal system, and comprises of pumps and sealed conduits through which hydrothermal fluid is circulated only between the loop and the reservoir, while heat is exchanged with the surroundings for electricity generation and other purposes [3].

4). Compost Vessel
Composting can be done in a closed container, and this is referred to as in-vessel composting (IVC), enclosed vessel composting, or closed composting (CC).
Enclosed vessel composting is an example of a closed thermodynamic system, in which the controlled biodegradation of organic biomass allows heat energy to be released to the environment, while all the organic matter remains confined within the vessel.
Types of in-vessel composting include inter-modal, silo-based, and drum-based composting, which are distinguished based on the designs and types of vessels involved.
In addition to being an example of closed systems, compost vessels can serve as an effective tool for recycling organic waste [1].
The disadvantage of in-vessel composting is its potential environmental impacts, which include greenhouse emissions and air pollution.
5). The Earth (as one of the Examples of Closed Systems)
The Earth is a closed system, because it exchanges energy in the form of solar radiation, with the outer space, but retains all its matter by the use of gravitational force fields.
Solar energy that the Earth receives from the Sun, serves as a primary energy, that is converted into other types of energy that can be found on the Earth's surface.
The Earth in turn releases solar radiation from its surface to the upper atmosphere and outer space. This mechanism helps to maintain a state of thermodynamic equilibrium between the Earth and other planetary bodies in the solar system.
Conclusion
Examples of closed systems are;
1. Sealed Heater
2. Water Bottle
3. Subsurface Hydrothermal Reservoir
4. Compost Vessel
5. The Earth
References
1). Kabbashi, N. A.; Opatokun, S. A.; Alam, Z.; Mirghani, M. E..S. (2014). "Recycling of organic wastes using locally isolated lignocellulolytic strains and sustainable technology." Journal of Material Cycles and Waste Management 17(4). Available at: https://doi.org/10.1007/s10163-014-0309-z. (Accessed 6 March 2023).
2). Mohsenian, H.; Ghadamian, H.; Hamidi, A. A.; Alizad, K. (2015). "PowerEnergy2015-49702 Expansion Tank Structural Reconstruction for Central Heating Systems with Re-Engineering Consideration and Energetic Losses Minimization." ASME 2015 9th International Conference on Energy Sustainability collocated with the ASME 2015 Power Conference, the ASME 2015 13th International Conference on Fuel Cell Science, Engineering and Technology, and the ASME 2015 Nuclear Forum, San Diego, CAVolume: V002T18A009-V002T18A009. Available at: https://doi.org/10.1115/ES2015-49702. (Accessed 6 March 2023).
3). Perego, R.; Viesi, D.; Pera, S.; Santa, G. D.; Cultrera, M.; Visintainer, P.; Galgaro, A. (2020). "Revision of hydrothermal constraints for the installation of closed-loop shallow geothermal systems through underground investigation, monitoring and modeling." Renewable Energy 153(3). Available at: https://doi.org/10.1016/j.renene.2020.02.068. (Accessed 6 March 2023).
4). Shalihin, G. J.; Utami, P.; Nurpratama, M. I. (2020). "The Subsurface Geology and Hydrothermal Alteration of the Dieng Geothermal Field, Central Java: A Progress Report." IOP Conference Series Earth and Environmental Science 417(1):012010. Available at: https://doi.org/10.1088/1755-1315/417/1/012010. (Accessed 6 March 2023).
Examples of closed systems are; sealed heater, water bottle, subsurface hydrothermal reservoir, compost vessel, and the Earth.
This article discusses the examples of closed systems, as follows;
1). Sealed Heater (as one of the Examples of Closed Systems)
Sealed heater, also called sealed heating system, sealed central heating system or sealed boiler, is a classic example of a thermodynamic closed system.
A sealed heating system is a system for generating and emitting thermal energy, whose physical boundaries seclude its fluid content from the environment or atmosphere.
From the above it can be deduced that a heating system is a closed system, when its internal mass remains constant at all times.
The difference between sealed and unsealed heating systems (also called open-vented systems) is that the former is equipped to regulate its fluid pressure internally, while the latter is de-pressurized by emitting its fluid content into an external system.
This external body in open-vented heating systems is the feed and expansion tank, which exchanges working fluid with the main heater to ensure that pressure is regulated for optimal energy efficiency and performance [2].
In the sealed boiler/heating system, an expansion vessel is also present, however it is installed so that it also stays within the confines of the main heater. When the working fluid in the boiler is heated and pressure increases, this pressure (along with temperature) is regulated by the migration (not release) of the vaporized working fluid into the expansion chamber.
The setup is very useful where water conservation (or conservation of any other working fluid) is necessary, as it ensures that fluid is not lost by the heating system at any time. It is also useful where simplicity and minimal maintenance are core objectives.
However, because of its restricted working conditions, the sealed heating system is not as efficient as open-vented system for large-scale heating purposes.
Sealed heating system clearly illustrates the basic attributes of a closed system, in terms of matter/mass conservation in combination with effective energy transfer.
2). Water Bottle
A water bottle is an example of a closed system, when it is sealed and composed of conductive material.
The most classic form of this is a sealed metallic bottle containing fluid.
An ideal thermos bottle is not a closed system, but rather is an isolated system, because it neither exchanges thermal energy nor fluid-mass with its surroundings.
However, in real-life scenarios, a thermos bottle is a closed system, because over an extended time period, it eventually releases its thermal energy to the surroundings, so that its fluid content cools down.
A hot water bottle is a closed system, while a hot water bottle that leaks is an open system, because it releases both heat and matter (fluid) to the external environment.

3). Subsurface Hydrothermal Reservoir (as one of the Examples of Closed Systems)
A hydrothermal system is one which has both hydro energy and thermal energy.
Hydrothermal systems work using the relationship between physical factors like pressure and temperature, to control the dynamics of fluids.
Characteristics of hydrothermal systems include significant fluid content, thermal energy, and definite physical boundaries.
The different types of hydrothermal systems are; subsurface, surface, open, closed, high and low-temperature hydrothermal systems.
The difference between hydrothermal and geothermal systems is that the primary components of hydrothermal systems are water and heat; while geothermal systems include water, heat, and earth materials like rocks, as primary components.
A hydrothermal system or reservoir can be described as a closed system, when it prevents the emission or introduction of water in any of its phases (liquid, gas, solid), across the physical boundaries of the reservoir.
The above describes a subsurface hydrothermal reservoir. Being underground, the physical boundaries of such a geologic system comprises of rocks and fluids, which allow heat to be transferred out of the reservoir, even to the surface.
Energy transfer for the subsurface hydrothermal reservoir occurs by conduction, radiation and convection. On the other hand, matter is not transferred, for as long as the reservoir is overlain and underlain by impervious rock materials.
The thermal energy in subsurface hydrothermal reservoirs is gotten primarily from magma [4], which in turn gains energy from nuclear radioisotopes in the Earth's core.
A subsurface hydrothermal reservoir becomes an open system when its physical boundaries are breached. This can occur due to human activities like the drilling of geothermal wells for power plants.
After drilling a well into the natural hydrothermal reservoir, a manmade closed system can still be created using fluid-pumping technology.
This is alternatively called a closed-loop geothermal system, and comprises of pumps and sealed conduits through which hydrothermal fluid is circulated only between the loop and the reservoir, while heat is exchanged with the surroundings for electricity generation and other purposes [3].
Examples of Closed Systems: Subsurface Hydrothermal Reservoir (Credit: Goran tek-en 2014 .CC BY-SA 4.0.)
4). Compost Vessel
Composting can be done in a closed container, and this is referred to as in-vessel composting (IVC), enclosed vessel composting, or closed composting (CC).
Enclosed vessel composting is an example of a closed thermodynamic system, in which the controlled biodegradation of organic biomass allows heat energy to be released to the environment, while all the organic matter remains confined within the vessel.
Types of in-vessel composting include inter-modal, silo-based, and drum-based composting, which are distinguished based on the designs and types of vessels involved.
In addition to being an example of closed systems, compost vessels can serve as an effective tool for recycling organic waste [1].
The disadvantage of in-vessel composting is its potential environmental impacts, which include greenhouse emissions and air pollution.
5). The Earth (as one of the Examples of Closed Systems)
The Earth is a closed system, because it exchanges energy in the form of solar radiation, with the outer space, but retains all its matter by the use of gravitational force fields.
Solar energy that the Earth receives from the Sun, serves as a primary energy, that is converted into other types of energy that can be found on the Earth's surface.
The Earth in turn releases solar radiation from its surface to the upper atmosphere and outer space. This mechanism helps to maintain a state of thermodynamic equilibrium between the Earth and other planetary bodies in the solar system.
Conclusion
Examples of closed systems are;
1. Sealed Heater
2. Water Bottle
3. Subsurface Hydrothermal Reservoir
4. Compost Vessel
5. The Earth
References
1). Kabbashi, N. A.; Opatokun, S. A.; Alam, Z.; Mirghani, M. E..S. (2014). "Recycling of organic wastes using locally isolated lignocellulolytic strains and sustainable technology." Journal of Material Cycles and Waste Management 17(4). Available at: https://doi.org/10.1007/s10163-014-0309-z. (Accessed 6 March 2023).
2). Mohsenian, H.; Ghadamian, H.; Hamidi, A. A.; Alizad, K. (2015). "PowerEnergy2015-49702 Expansion Tank Structural Reconstruction for Central Heating Systems with Re-Engineering Consideration and Energetic Losses Minimization." ASME 2015 9th International Conference on Energy Sustainability collocated with the ASME 2015 Power Conference, the ASME 2015 13th International Conference on Fuel Cell Science, Engineering and Technology, and the ASME 2015 Nuclear Forum, San Diego, CAVolume: V002T18A009-V002T18A009. Available at: https://doi.org/10.1115/ES2015-49702. (Accessed 6 March 2023).
3). Perego, R.; Viesi, D.; Pera, S.; Santa, G. D.; Cultrera, M.; Visintainer, P.; Galgaro, A. (2020). "Revision of hydrothermal constraints for the installation of closed-loop shallow geothermal systems through underground investigation, monitoring and modeling." Renewable Energy 153(3). Available at: https://doi.org/10.1016/j.renene.2020.02.068. (Accessed 6 March 2023).
4). Shalihin, G. J.; Utami, P.; Nurpratama, M. I. (2020). "The Subsurface Geology and Hydrothermal Alteration of the Dieng Geothermal Field, Central Java: A Progress Report." IOP Conference Series Earth and Environmental Science 417(1):012010. Available at: https://doi.org/10.1088/1755-1315/417/1/012010. (Accessed 6 March 2023).