16 Environmental Remediation Techniques Explained
Environmental remediation techniques are; nanoremediation, thermal desorption, oxidation, reduction, microbial biodegradation, bioreactor usage, biowall usage, electrokinetics, pump and treat, permeable reactive barrier, soil washing, air sparging, vitrification, encapsulation, multiphase extraction, phytoremediation, and surfactant enhanced remediation.
These techniques can be broadly grouped into three groups; physical, chemical and biological remediation techniques.
In this article, the techniques of environmental remediation are discussed, according to the outline below;
-Physical Environmental Remediation Techniques
-Chemical Environmental Remediation Techniques
-Biological Environmental Remediation Techniques
-Physical Environmental Remediation Techniques
Physical techniques of environmental remediation are techniques which mainly involve physical mechanisms and processes.
They include air sparging, permeable barrier usage, electrokinetics, encapsulation, thermal desorption, vitrification, and pump-and-treat.
These techniques all depend on physical properties and processes, to be effective.
1). Air Sparging
Air sparging is a physical technique of environmental remediation, which involves the injection of air under high pressure, into a contaminated medium, to create a pressure and concentration gradient that can help to remove pollutants through the process of migration.
It is an in-situ technique, which is alternatively referred to as ‘in situ air stripping’ or ‘in situ air volatilization’.
The technique is usually applied in cases of groundwater pollution [14]. This is because polluted groundwater provides a confined, multiphase medium which can be altered significantly by air sparging.
The primary goal of injecting air into the contaminated medium in this technique, is to create a disruption of phase equilibrium, which can help to segregate the contaminants due to differences in physical attributes like density and solubility.
Air sparging is often utilized in the treatment of groundwater that is contaminated by volatile organic compounds (VOCs) [6]. The injection of high-pressure air into a medium containing such contaminants, causes their removal through volatilization.
In addition to volatilizing pollutants, air sparging helps to facilitate the breakdown of pollutants, by supplying the needed oxygen for aerobic biodegradation [15]. This means that the technique can act as a supportive measure to bioremediation.
Air sparging is also used in combination with other environmental remediation techniques, like soil vapor extraction [2]. In such cases, air sparging is used to facilitate the removal of pollutants by creating a concentration gradient in the polluted medium.
2). Permeable Barrier Usage
Also known as a ‘permeable reactive barrier (PRB);’ the use of permeable barriers is a physicochemical technique of environmental remediation, that utilizes both physical and chemical characteristics to remove pollutants from a medium.
It is also a passive remediation technique [11], because it does not require continuous input of energy or time throughout the remedial process.
Permeable reactive barrier usage is best for groundwater treatment, and is an in-situ method, meaning that it does not require the polluted medium to be removed from the site of pollution.
In order to be effective, the technique relies on physical and chemical factors such as fluid flow rate, density, temperature, pressure, volatility of pollutant(s), and chemical composition of pollutants.
Basically, a permeable reactive barrier system comprises of a permeable barrier or wall which is installed within the flow pathway of contaminated groundwater (in the subsurface), having the capacity to filter undissolved contaminants, while reacting with and breaking-down dissolved contaminants.
It is possible to compare the mechanism behind this technique to that which is used in reverse osmosis systems for water filtration.
The reactive nature of the barrier facilitates chemical reactions which breakdown and convert the contaminants in the water, while the permeability of the barrier allows the water to flow through, and at the same time, stops undissolved particles from flowing through.
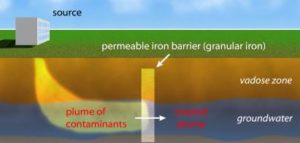
It is necessary for the permeable barrier to intercept the groundwater flow pathway in a downstream direction, in order for it to be effective. It is also necessary for the dissolved pollutants to be susceptible to conversion, through reaction with the barrier.
Some groundwater contaminants which can be addressed using this technique include ammonium, chlorides, organic compounds like toluene, and leachate containing heavy metals like cadmium.
3). Electrokinesis
Electrokinetics, or electrokinetic remediation is a technique that uses low-voltage, direct current to mobilize and remove contaminants from a medium, through the influence of an electric field on these contaminants.
The principle behind this method can be referred to as ‘electrokinetics’ or ‘electrohydrodynamics’, which is the movement of a fluid or particle by an electric field [5].
Predictably, the effectiveness of this technique depends on the presence of electrons, the strength of the electric field, and the conductivity of the contaminant materials.
Although electrons are part of the basic chemical structure of an atom, electrokinetics is a physical method of environmental remediation because it depends on physical characteristics like conductivity, and does involve any notable chemical reactions.
Electrokinetic remediation is also an in situ technique [7]. It does not require the removal of contaminated materials from the site of contamination.
Environmental degradation cases involving soil, are most suitable for electrokinetic remediation, because soil provides a favorable medium for the transmission of the direct current, and the full effect of the electric field.
Some terms used to describe the removal of pollutants using this technique, are electromigration and electro-osmosis [26].
Because electrokinetic remediation depends on electric conductivity, it is most effective for cases where the contaminants are fairly conductive. This includes cases where the contaminants are heavy metals or organic compounds [17].
4). Encapsulation
Encapsulation is an environmental remediation technique that involves the separation and immobilization of contaminants, to prevent their further spread within a given medium.
As the description above implies, this technique does not necessarily involve the removal of the contaminant. Rather, the goal in encapsulation is to ensure that the contaminant is confined within a restricted area, such that the degree of environmental degradation is not worsened.
Silica is a material that is commonly used for encapsulation [7] because of its resistance to physicochemical changes, and its compatibility with other earth materials.
The scale of encapsulation can be described as micro-encapsulation or macro-encapsulation, and the technique is best suited to cases of soil contamination.
Contaminants that can be addressed using encapsulation include polychlorinated biphenyls, heavy metals, and hydrocarbons.
5). Thermal Desorption
Thermal desorption is an environmental remediation technique whereby a contaminated medium is subjected to heating under controlled conditions, in order to vaporize and remove contaminants.
This technique is classified as a physical technique, because it does not necessarily involve any chemical disintegration or biological degradation of the contaminant.
Rather, thermal desorption solely depends on the physical attributes of the contaminant, and the physical conditions of the contaminated medium.
Thermal desorption is typically used to treat contaminated soil [30] because soil is a suitable medium for the effective application of heat to vaporize contaminants.
The technique can be used either in situ or ex situ, although it is more often used under ex situ conditions.
Based on the amount of heat that is applied, thermal desorption can be classified as low-temperature thermal desorption (LTTD) or high-temperature desorption (HTTD).
When the contaminants are vaporized by heat, the vapor is usually condensed and collected for disposal.
Contaminants that can be removed using thermal desorption include polycyclic aromatic hydrocarbons (PAHs), and heavy metals [22].

6). Pump and Treat
Pump-and-treat is an environmental remediation technique that involves the retrieval of contaminated groundwater from the subsurface by pumping, in order to subsequently treat this contaminated groundwater.
There are three main steps in the entire process of pump and treat remediation.
These are extraction, treatment and discharge.
From the description so far, it is possible to deduce that pump and treat is an ex situ technique of environmental remediation. It has also been implied that this technique is best suited for groundwater remediation projects.
During extraction (or ‘pumping’), the contaminated groundwater is withdrawn from the subsurface using designated extraction wells [16].
This extraction process ensured that the contaminated material is removed from the site, in order for effective treatment to be carried out.
Pump and treat method is usually applied when the level and/or spread-rate of contamination is high, and it can be used to extract non-aqueous phase liquids (NAPLs) when these contaminants are segregated from the main body of groundwater.
Treatment stage involves the removal or reduction of contaminants in the water that has been extracted. Any suitable method may be used to achieve this purpose, which implies that the pump and treat technique is applied in combination with other environmental remediation technologies.
In some cases, air sparging can be used to segregate the contaminant (or the zone of contamination) before pumping is done.
After the water has been treated, it can be discharged into the environment without any significant risks.
7). Vitrification
Vitrification is the treatment of contaminated sites by applying high temperature, to melt and solidify the contaminated medium.
This technique is mostly applied in cases of soil contamination [28]. When applied, the contaminated soil is subjected to intense thermal treatment, such that the zone of contamination is vitrified by melting and solidification.
The end-product of vitrification usually has glass-like characteristics, hence the term ‘vitrify’ from vitreous which also implies glassy.
The source of heat energy for vitrification may vary. In several cases, the heat is produced from electricity [10].
Vitrification is an applicable measure in highly-industrialized areas, and in cases where the contaminants are not easily addressed by other techniques.
In order for vitrification to be effective, the earth materials involved, must be susceptible to melting and recrystallization under high temperature.
It is also important to note that the goal of this technique is to immobilize contaminants, while rendering them less-hazardous.
Vitrification is generally an in-situ technique, and is prominently used to treat cases of pollution by radioactive materials [18].
-Chemical Environmental Remediation Techniques
Chemical techniques of environmental remediation, are techniques which make use of chemical properties, reactions and processes, to address cases of environmental degradation.
Examples of chemical remediation techniques include surfactant-enhanced remediation, reduction/oxidation, chemical leaching, hydrolysis, soil amendment/chemical fixation, and nanoremediation.
1). Surfactant-Enhanced Remediation
Surfactant-enhanced remediation (SER) is a technique of environmental remediation, whereby a surfactant is applied to a contaminated medium, in order to increase the solubility of the contaminants.
As the description above implies, surfactant-enhanced remediation is used mainly for contaminants that are hydrophobic and which do not dissolve easily [24].
It is essentially an in situ technique (although it may also be applied ex situ), and has advantages such as low cost and short duration of treatment, compared to other remediation techniques.
In some studies, the technique may be categorized as a physical technique, however, surfactant application and solubilization of contaminants, is always accompanied by some chemical reactions.
A common scenario of application of surfactant-enhanced remediation, is in groundwater treatment. In such scenarios, the surfactant is usually injected into the groundwater body, which may contain an insoluble contaminant like a non-aqueous phase liquid.
Often, the purpose of this technique is to prepare the site for application of another remediation technique; such as pump and treat. The goal is to make the contaminant soluble, so that it can be easily accessed in the contaminated water.
Surfactant-enhanced remediation can also be considered a biological remediation technique, because of the ability of microbes to produce surfactant materials, called biosurfactants [23].
2). Chemical Reduction and Oxidation
Reduction/Oxidation is one of the most common, chemical techniques for environmental remediation.
It involves the application of reducing or oxidizing agents, to a degraded environment, in order to convert contaminants to less-harmful products.
Both reduction and oxidation, lead to the formation or precipitation of compounds or free radicals, that are less toxic than their parent materials.
In the case of oxidation, the technique can be used for remediation of sites where heavy metal contamination has occurred [29]. When applied, oxidizing agents may convert these contaminants to precipitates like metallic sulfides, including lead sulfide (PbS) and iron sulfide (FeS).
Reduction can be used to treat sites contaminated by chlorinated organic compounds, and typically converts these contaminants to less-harmful products like ethene, methane and ethene.
Reduction/Oxidation is also generally applied in situ.
3). Chemical Leaching
Chemical leaching is a technique of environmental remediation that involves the use of chemical reagents to wash and extract toxic pollutants from a medium.
Also referred to as ‘soil leaching,’ this technique is usually applied to polluted soils.
Chemical leaching can be carried out either in situ or ex situ. It is similar to chemical desorption by relying on chemical reactions to increase the mobility of contaminants.
The mechanism of chemical leaching usually involves dissolution, extraction and separation of pollutants.
Some contaminants that can be addressed using this technique include compounds of heavy metals like Chromium and Cadmium.
The main disadvantage of this technique is its capacity to negatively affect soil fertility [9].
4). Hydrolysis
Hydrolysis is a chemical reaction in which chemical bonds in a compound are broken due to the splitting of a water molecule [20].
This reaction can be used in environmental remediation, as a technique whereby a contaminant is broken down by reason of hydrolysis, with the simultaneous splitting of water molecules.
Hydrolysis can also be described as a process of chemical decomposition, which involves the splitting of bonds and the addition of hydrogen and hydroxyl ions.
One of the common scenarios where hydrolysis is applied, is in the treatment of contaminated soil. In such cases, the remediation process involves raising the pH of the soil to facilitate the production of ions, the splitting of water molecules, and the breakdown of contaminants.
Measures which may be taken to increase the effectiveness of hydrolysis include the application of thermal energy and electricity (12].
Hydrolysis is used in remediation of environments that contain contaminants like toluene and trichloroethane.
5). Soil Amendment/Chemical Fixation
Soil amendment and chemical fixation are closely related environmental remediation techniques, which involve the addition of chemical reagents to a medium, to either immobilize contaminants, or improve quality.
With regards to improving quality, soil amendment is solely designed to achieve this purpose.
Soil amendment refers to a material or process that is applied to soil, to improve its quality. This implies that soil amendment may be used to refer to a soil additive, or the practice of applying a soil additive.
As the definition implies, the purpose of soil amendment is to improve the quality of soil.
This ‘quality’ can be defined in terms of different factors, such as absorption/retention capacity, nutrient content, cohesiveness, pH, structure, aeration, and drainage.
Soil amendments (additives) can be organic or inorganic [4]. Examples of soil amendments include organic biomass such as animal waste and plant waste; as well as inorganic materials like ash, lime, coal, perlite, quartz, and vermiculite.
Cases where soil amendment is applied include soil preparation for agricultural purposes, and soil restoration in mine sites.

Chemical fixation is the application of chemical substances to a contaminated medium, in order to immobilize the contaminants and prevent further spread.
The technique is commonly used in remediation of soils contaminated by heavy metals [19].
Additives used in chemical fixation, convert contaminants into less-toxic, insoluble and relatively immobile materials.
Examples of such additives include; ethanol, acetone and methanol.
Both soil amendment and chemical fixation are classified as chemical techniques of environmental remediation, because they involve chemical changes in soil composition, and conversion of contaminants into less-harmful materials.
6). Nanoremediation
Nanoremediation is a technique of environmental remediation which uses nanomaterials (comprised of nano-sized particles) to convert contaminants to less-harmful materials, through chemical reactions.
The technique can be used to treat contaminated soil, water and air (Hussain et al. 2021).
Nanoremediation has the advantage of being a relatively safe and cost-effective technique, which is effective for cases involving petroleum, chlorinated solvent and pesticide pollution.
-Biological Environmental Remediation Techniques
Biological techniques of environmental remediation, are techniques which are driven by biological organisms, substances and processes.
This technique is otherwise referred to as bioremediation.
The two main techniques of bioremediation are microbial bioremediation and phytoremediation.
1). Microbial Bioremediation
Microbial bioremediation is the use of microorganisms to breakdown contaminants in a medium, through the process of biodegradation.
The purpose of microbial bioremediation is to convert contaminants into less-toxic or non-toxic materials that do not pose significant harm to the environment [25].
It has some attributes of a chemical remediation technique, because it involves chemical reactions and conversions. However, the prominence of organisms and organic processes gives it the classification of a biological remediation technique.
Various microbes can be used for microbial bioremediation. These include bacteria, fungi, and algae.
Some conditions are required for microbial remediation to be effective. These include the presence of biodegradable contaminants, suitable microbial populations, and favorable environmental conditions like temperature, humidity and pH.
Petroleum contamination is an example of a scenario where microbial remediation can be applied effectively.
2). Phytoremediation
Phytoremediation is a biological technique of environmental remediation, whereby plants and plant-related microorganisms are used to stabilize, breakdown, transfer or extract contaminants from soil.
The above definition implies that phytoremediation is solely used for soil. However, the technique can be used to treat contaminated surface water and groundwater as well.
Phytoremediation works based on the ability of plants to concentrate and extract minerals in the process of their nutrition and growth.
Where the contaminants contain some minerals required by plants, these plants eliminate or reduce the contaminants in the process of extracting these minerals.
Two major advantages of phytoremediation are its cost-effectiveness and its environmental friendliness [21].
Aside conventional cases of environmental degradation, phytoremediation can also be used to address issues of eutrophication, by extracting the excessive nutrients that are present in water [3].
Various types of plants including trees, shrubs and aquatic plants, can be used for phytoremediation. Examples of contaminants that can be addressed using this technique ae pesticides and heavy metals.
3). Biowall and Bioreactor Usage
Biowall and bioreactor are technologies that make use of biological organisms and processes, for environmental remediation.
A biowall is similar to a permeable reactive barrier (PRB), but is also equipped with tools to facilitate anaerobic biodegradation.
In general, a biowall is equipped with organic mulch [1]; which is comprised of biomass, or biopoymers, microbes and enzymes, that come in contact with contaminants and cause biodegradation to occur.
The biowall is usually placed in the flow pathway of contaminated groundwater. Polyaromatic hydrocarbons (PAHs) represent a group of contaminants that can be addressed using this technology.
Bioreactors are like biowalls in the sense that they facilitate environmental remediation through biodegradation. However, while a biowall is a barrier, a bioreactor is usually a vessel, into which contaminated material is poured.

Contaminated soil and groundwater can both be treated using bioreactors [27], and the technology is suitable for removing petroleum pollutants and volatile organic compounds.
Bioreactors are used in ex situ environmental remediation, to provide suitable environmental conditions for the breakdown of contaminants.
Conclusion
Environmental Remediation Techniques are;
1. Air Sparging
2. Permeable Barrier Usage
3. Electrokinetics
4. Encapsulation
5. Thermal Desorption
6. Pump and Treat
7. Vitrification
8. Surfactant-Enhanced Remediation
9. Chemical Reduction and Oxidation
10. Chemical Leaching
11. Hydrolysis
12. Soil Amendment/Chemical Fixation
13. Nanoremediation
14. Microbial Bioremediation
15. Phytoremediation
16. Biowall and Bioreactor Usage
These techniques can be broadly classified as physical, chemical and biological, based on the mechanisms and processes involved.
References
1). Ahmad, F.; McGuire, T. M.; Lee, R. S.; Becvar, E. (2007). “Considerations for the design of organic mulch permeable reactive barriers.” Remediation Journal 18(1):59 – 72. Available at: https://doi.org/10.1002/rem.20151. (Accessed 12 April 2022).
2). Al-Maamari, R. S.; Hirayama, A.; Sueyoshi, M. N.; Abdalla, O.; Al-Bemani, A.; Islam, M. R. (2021). “The Application of Air-Sparging, Soil Vapor Extraction and Pump & Treat for Remediation of a Diesel-Contaminated Fractured Formation.” Energy Sources, Part A: Recovery, Utilization and Environmental Effects 31(11). Available at: https://doi.org/10.1080/15567030801904236. (Accessed 12 April 2022).
3). Ansari, A. A.; Gill, S. S.; Khan. F. A.; Naeem, M. (2014). “Phytoremediation systems for the recovery of nutrients from eutrophic waters.” In book: Eutrophication: Causes, Consequences and Control, Volume-2 (pp.239-248), Springer. Available at: https://www.researchgate.net/publication/266599090_Phytoremediation_systems_for_the_recovery_of_nutrients_from_eutrophic_waters. (Accessed 12 April 2022)..
4). Antonious, G. F. (2016). “Soil Amendments for Agricultural Production”, In M. L. Larramendy, S. Soloneski (eds.), Organic Fertilizers – From Basic Concepts to Applied Outcomes, IntechOpen, London. Available at: https://doi.org/10.5772/63047. (Accessed 12 April 2022)..
5). Bazant, M. Z. (2015). “Electrokinetics meets electrohydrodynamics.” Journal of Fluid Mechanics 782:1-4. Available at: https//doi.org/10.1017/jfm.2015.416. (Accessed 12 April 2022).
6). Braida W. J.; Ong S. K. (2001). “Air sparging effectiveness: laboratory characterization of air-channel mass transfer zone for VOC volatilization.” J Hazard Mater. 2001 Oct 12;87(1-3):241-58. Available at: https://doi.org/10.1016/s0304-3894(01)00287-4. (Accessed 12 April 2022).
7). Camenzuli, D.; Gore, D. (2013). “Immobilization and Encapsulation of Contaminants Using Silica Treatments: A Review.” Remediation Journal 24(1). Available at: https://doi.org/10.1002/rem.21377. (Accessed 12 April 2022).
8). Cameselle, C.; Gouveia, S.; Akretche, D. E.; Belhadj, B. (2013). “Advances in Electrokinetic Remediation for the Removal of Organic Contaminants in Soils.” In (Ed.), Organic Pollutants – Monitoring, Risk and Treatment. IntechOpen. Available at: https://doi.org/10.5772/54334. (Accessed 12 April 2022).
9). Chu, C.; Ko, T. (2018). “Evaluation of Acid Leaching on the Removal of Heavy Metals and Soil Fertility in Contaminated Soil”, Journal of Chemistry, vol. 2018, Article ID 5036581, 8 pages,2018. Available at: https://doi.org/10.1155/2018/5036581. (Accessed 12 April 2022).
10). Dellisanti, F.; Rossi, P. L.; Valdre, G. (2009). “Infield remediation of tons of heavy metal-rich waste by Joule heating vitrification.” International Journal of Mineral Processing 93(3):239-245. Available at: https://doi.org/10.1016/j.minpro.2009.09.002. (Accessed 12 April 2022).
11). Faisal, A.; Sulaymon, A. H.; Khaliefa, Q. (2017). “A review of permeable reactive barrier as passive sustainable technology for groundwater remediation.” International journal of Environmental Science and Technology 15(4). Available at: https://doi.org/10.1007/s13762-017-1466-0. (Accessed 12 April 2022).
12). García-Cascallana, J.; Borge-Díez, D; Gómez, K. (2018). “Enhancing the efficiency of thermal hydrolysis process in wastewater treatment plants by the use of steam accumulation.” International journal of Environmental Science and Technology 16(10):1-16. Available at: https://doi.org/10.1007/s13762-018-1982-6. (Accessed 12 April 2022).
13). Hussain, A.; Rehman, F.; Rafeeq, H.; Waqas, M.; Ashgar, A.; Afsheen, N.; Rahdar, A.; Bilal, M.; Iqbal, H. (2021). “In-situ, Ex-situ, and nano-remediation strategies to treat polluted soil, water, and air – A review.” Chemosphere 289(1–3):133252. Available at” https://doi.org/10.1016/j.chemosphere.2021.133252. (Accessed 12 April 2022).
14). Johnson, R.; Johnson, P. C.; McWhorter, D. B.; Hinchee, R.; Goodman, I. (2007). “An Overview of In Situ Air Sparging.” Ground Water Monitoring and Remediation 13(4):127 – 135. Available at: https://doi.org/10.1111/j.1745-6592.1993.tb00456.x. (Accessed 12 April 2022).
15). Kasanke, C. P.; Willis, M. D.; Leigh, M. R. (2021). “Distribution of a Sulfolane-Metabolizing Rhodoferax sp. Throughout a Contaminated Subarctic Aquifer and Two Groundwater Treatment Systems.” Front. Microbiol., 26 August 2021. Available at: https://doi.org/10.3389/fmicb.2021.714769. (Accessed 12 April 2022).
16). Krishnakumari, B.; Gayathiri, Dhivya , B.; Abarna, K. (2018). “Remediation of Contaminated Ground Water.” INTERNATIONAL JOURNAL OF ENGINEERING RESEARCH & TECHNOLOGY (IJERT) PECTEAM – 2018 (Volume 6 – Issue 02). Available at: http://dx.doi.org/10.17577/IJERTCON018. (Accessed 12 April 2022).
17). Lukman, S.; Essa, M. H.; Mu’azu, N. D.; Bukhari, A. (2013). “Coupled Electrokinetics-Adsorption Technique for Simultaneous Removal of Heavy Metals and Organics from Saline-Sodic Soil”, The Scientific World Journal, vol. 2013, Article ID 346910, 9pages, 2013. Available at: https://doi.org/10.1155/2013/346910. (Accessed 12 April 2022).
18). Marra, J. C.; Ojovan, M. I. (2014). “Vitrification of radioactive wastes.” Available at: https://www.researchgate.net/publication/290189161_Vitrification_of_radioactive_wastes. (Accessed 12 April 2022).
19). Nejad, Z. D.; Jung, M. C.; Kim, K. (2018). “Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology.” Environmental Geochemistry and Health 40(1–2). Available at: https://doi.org/10.1007/s10653-017-9964-z. (Accessed 12 April 2022).
20). Phillips, T. (2019). “An Explanation of the Process of Hydrolysis.” Available at: https://www.thoughtco.com/what-is-hydrolysis-375589. (Accessed 12 April 2022).
21). Razzaq, R. (2017). “Phytoremediation: An Environmental Friendly Technique – A Review.” International Journal of Environmental Analytical Chemistry 04(02). Available at: https://doi.org/10.4172/2380-2391.1000195. (Accessed 12 April 2022)..
22). Saeedi, M.; Li, L.; Grace, J. (2020). “Effect of Co-existing Heavy Metals and Natural Organic Matter on Sorption/Desorption of Polycyclic Aromatic Hydrocarbons in Soil: A Review.” Pollution, 6(1), 1-24. Available at: https://doi.org/10.22059/poll.2019.284335.638. (Accessed 12 April 2022).
23). Sáenz-Marta, C. I. , de Lourdes Ballinas-Casarrubias, M., Rivera-Chavira, B. E. , & Nevárez-Moorillón, G. V. (2015). “Biosurfactants as Useful Tools in Bioremediation.” In (Ed.), Advances in Bioremediation of Wastewater and Polluted Soil. IntechOpen. Available at: https://doi.org/10.5772/60751. (Accessed 12 April 2022).
24). Saint-Fort, R. (2021). “Surfactants and Their Applications for Remediation of Hydrophobic Organic Contaminants in Soils.” in A. K. Dutta (ed.), Surfactants, IntechOpen, London. Available at: https://doi.org/10.5772/intechopen.100596. (Accessed 12 April 2022).
25). Sharma, I. (2020). “Bioremediation Techniques for Polluted Environment: Concept, Advantages, Limitations, and Prospects”, in M. A. Murillo-Tovar, H. Saldarriaga-Noreña, A. Saeid (eds.), Trace Metals in the Environment – New Approaches and Recent Advances, IntechOpen, London. Available at: https//doi.org/10.5772/intechopen.90453. (Accessed 12 April 2022).
26). Shenbagavalli, S.; Mahimairaja, S. (2010). “Electro kinetic remediation of contaminated habitats.” African Journal of Environmental Science and Technology Vol. 4 No. 13 (2010): Special Issue. Available at: https://www.ajol.info/index.php/ajest/article/view/71410. (Accessed 12 April 2022).
27). Tekere, M. (2019). “Microbial Bioremediation and Different Bioreactors Designs Applied“, in E. J. -Lopes, L. Q. Zepka (eds.), Biotechnology and Bioengineering, IntechOpen, London. Available at: https://doi.org/10.5772/intechopen.83661. (Accessed 12 April 2022).
28). Trifunović, V. (2021). “Vitrification as a method of soil remediation.” Zastita Materijala 62(3):166-179. Available at: https://doi.org/10.5937/zasmat2103166T. (Accessed 12 April 2022).
29). Yoo, C.; Lee, C.; Lee, J. S.; Baek, K. (2017). “Simultaneous application of chemical oxidation and extraction processes is effective at remediating soil Co-contaminated with petroleum and heavy metals.” J Environ Manage. 2017 Jan 15;186(Pt 2):314-319. Available at: https://doi.org/10.1016/j.jenvman.2016.03.016. (Accessed 12 April 2022).
30). Zhao, C.; Feng, Y.; Dong, Y. (2019). “Thermal desorption for remediation of contaminated soil: A review.” Chemosphere 221(1). Available at: https://doi.org/10.1016/j.chemosphere.2019.01.079. (Accessed 12 April 2022).